DAVID O’CAEIRO
Author

Audit Day Fears are Gone! How Cardio-Phoenix carried out of an ISO Audit With Help from qmsWrapper
Audit – a word that inspires anxiety. Even when everything is well planned, and you think you are prepared, anticipation turns to anxiety, anxiety to fear, and fear to panic. There is no hiding anything in a remote closet or under the carpet.
“Wherever you've hidden it, we'll find it.”
Audit Senior
It's an audit day!
In the beginning,...

How to stay on top of your product development and easily organize your FDA and CE submission
Traceability is required by regulations.
Traceability Matrix Updated 2019,
For FDA, 510(k), DeNovo or PMA, traceability is required as part of your Design History File (DHF). Soup to nuts, they expect you to demonstrate how it is all connected, where and how requirements were met.
For the CE mark, the requirements are similar, if not the same!
Failure to...

Pitfalls and Best Practices in Establishing a Quality Manual
It takes the same amount of time and effort to create a good quality manual as it does a bad one. Oftentimes, far less...
In this article, we will discuss how to establish, operate and maintain a good one:
Introduction (what is a QM, and what should include)
Common mistakes in establishing a QM, and how to avoid them
Pro...

Are MedDev companies ready for the ISO 13485:2016? “A Closer Look”
Surprisingly, this is a real and topical question. At every MedDev or MedTech oriented exhibition this is the off-stage topic, actually more like the main grippe session. The 2016 ISO version requires a lot more emphasis on risk management and embedding risk management into the quality system processes. But really there are two issues in play...

Do you know how secure your documents really are?
Data loss or damage is costly, extremely risky and can affect your brand reputation. Perils might come in many forms, as natural disasters (e.g. hurricanes, floods, fire) or uncontrollable human errors, power surges, technical malfunctions and increasingly malware attacks. You need a backup plan and the ability to restore your data quickly.
Warning steps through infographics
While...

Advantages of Cloud Based QMS and Project Management Software
Advantages in numbers
Every industry or organization is working hard to achieve success and to ensure their service and product safety. Nowadays, it is hard to imagine the work without an electronic, cloud-based QMS or project management tool.
Cloud-based tools are the most searched software today, they are easy to obtain through the internet, they are flexible,...

ISO 13485 – 2016 Checklist
A checklist on where to start, and what to do first
Whether you are freshly minted into the QMS position or you are a founder of a Startup or a product manager with a new project, you’re reading this because your strategy requires QMS oversight and your first question is likely “where to start” and “what...

The Purpose of a Quality Management System
Small businesses typically face a struggle to succeed in a competitive MedDev marketplace. Companies that offer quality services or products are better positioned to not only survive but prosper. A well-managed QMS is paramount to success.
What is a good Quality Management System?
QMS systems come in all flavors and shapes, some are industry-specific, others more general,...
Approval Workflows in QMS
Issues in approval workflows
Achieving and maintaining compliance is not an easy job as both ISO and FDA regulations require that documents be approved before they are officially distributed or used either inside or outside the company. Obtaining approval from a group of people for a project plan, a proposal, or any other required document can...
Balancing Priorities when everything is #1
Ballance as a secret to success
Every given task needs to have a clear priority. In the medical device industry, when everything seems so important, and you are not sure where to begin, it's crucial to collect all your tasks, identify them, maybe order them by estimated effort, etc..
Especially if you are a beginner, your to-do...
Documents in a Paperless Office, Find a Needle in the Haystack
The QMS documentation – especially for a complex project – can be endless and impenetrable. All of us are familiar with the pain of trying to make order out of complete file chaos, or the vain hope of finding a particular document in a pile of paperwork, be it old-school paper-based or modern electronic software...
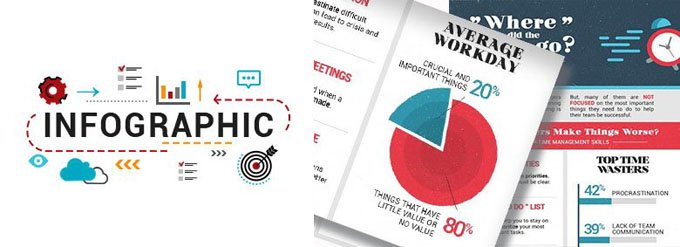
Where Did the Time Go? - Poor Time Management
One of the challenges managers face today is that all is happening in the present time and everything is urgent.
But, many of them are not focused on the most important things they need to do to help their team be successful.
How Managers Make Things Worse?
Good time management requires driving attention from activities to results: being...

Quality Manual or Workflow Processes? What should I establish first?
So, what documentation hierarchy should we adopt?
It turns out that this question is a classic for a reason. People have been asking it for many years, and there is more than a little confusion on the answer.
The Quality Manual requires many references to the applied company and the workflow processes. Synchronously, most quality management related...

QMS for Beginners – A quick guide on where to Start ! …Part 4
12. Customer Support and Life Cycle management
Customer support is not the issue at this time. You need to develop and finish your product first, then get it cleared through FDA and CE Approved. You need a system that’s focused on those issues. Don’t get confused by these future requirements. There is time enough to figure...

QMS for Beginners – A quick guide on where to Start ! …Part 3
The 13 items that will guide you through the implementation of QMS ...Let's pick up where we left off !
8. Selecting the right system to support your Compliance
Compliance Solutions are essentially QMS based solutions. They come in all shapes and sizes. Some solutions, such as ZenQMS are nothing more than forms tacked on to a project management...

QMS for Beginners – A quick guide on where to Start ! …Part 2
The 13 items that will guide you through the implementation of QMS - continues...
4. Organizing the work
Next you will need to define the flow of how work gets done within your organization, i.e. the workflows. Why, because you need to understand where compliance issues will fit in. Workflows can show you the path tasks take through your organization… i.e....

QMS for Beginners – A quick guide on where to Start ! …Part 1
Whether you are freshly minted into the QMS position or you are a founder of a Startup or a product manager with a new project, you’re reading this because your strategy requires QMS oversight and your first question is likely "Where to Start” and “What to Do”
We could squeeze in 5 main points the initial...

Project management and quality management 2017 - smart solution
qmsWrapper is 1 system with 5 modules
and if you thought project management couldn't include QMS... think again...
In a global environment, companies are facing countless complex and unique industry challenges. Quality becomes not just a buzzword but a true competitive advantage – companies that deliver the best quality products and services efficiently - succeed.
Today’s QMS and...

The Dawn of Integrated QMS… Part 2
qmsWrapper, the leading integrated QMS system specifically aimed at compliance
qmsWrapper has Compliance in its DNA.
Since developed by a medical device company, the priority was to design a QMS application that solves many challenges facing medical device startups and small companies. Based on Project, Quality, Risk, and Document Management with a truly comprehensive Team messaging. In qmsWrapper,...

The Dawn of Integrated QMS… Part 1
Making the life of a startup easier
As regulatory and standards compliance increasingly play a critical role in business success, integrated compliance tools remain a sore point. QMS systems are too often based on PDF documents being generated at the right time. The challenge for the user is knowing when that time is, and what form...

QMS Expert vs QMS Expert...alias - Today Everybody is an Internet Certified QMS Expert
The newbie QMS Managers Conundrum – 5 things to identify the right Expert?
As a newly minted QMS manager in a medical device startup, I turned to the internet for what to do. Soon I had to realize that today everybody is an internet certified QMS expert. There was information galore, in fact so much information...

Top Documentation Pain Points Solved With DM Software
Today’s businesses are up against more challenges than ever in the past. While some businesses think they don’t need to move away from paper, getting started with this practice has never been so crucial.
Document management software makes it easy
for businesses to combine paper and digital files into a single hub, as physical documents. Companies that...

Risk as synonymous with uncertainty?
” Risk /rɪsk/, noun
the possibility that something unpleasant or unwelcome will happen.
Synonyms: chance, uncertainty, unpredictability, instability, riskiness…”
We’re dealing with countless risks in our daily life, like getting cut during apple slicing, or catching a cold if we get wet on a rainy day…or planning a project without a proper risk management plan…
Unknowingly you are doing...

Chat for Compliance – a hacker’s view!
This week we bring you a real-life experience example about how the
qmsWrapper built-in chat
function can turn the compliance documenting process into an easily manageable one.
The ongoing quest for compliance can sometimes be challenging when the needs are not always clear. For example, developing new software is straightforward, customer needs or use cases, requirements, specification, test...
ISO 13485:2016 Summary
Medical Devices – Quality Management System – Requirements for Regulatory Purposes
The world’s most popular standard for medical device quality management has been revised for the first time since 2003: Medical Devices – Quality Management System – Requirements for Regulatory Purposes.
The revised standard ISO 13485:2016 was published on 1st March 2016. It focuses on how companies...