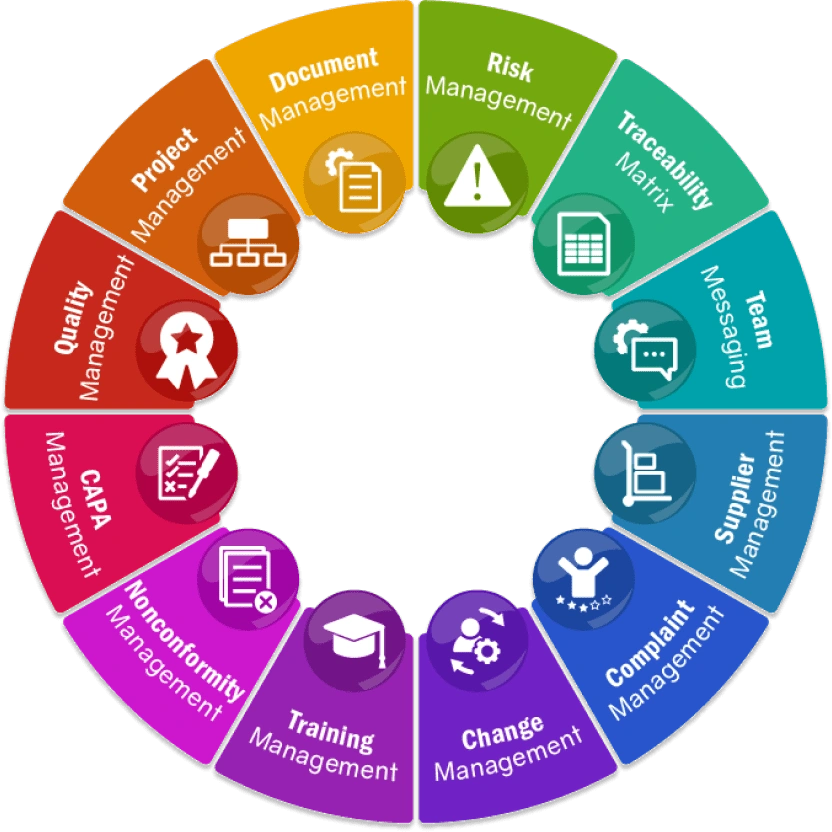
QMS Features & Modules
Discover an all-in-one QMS platform for projects, documents
and risk—now with Event & Hazard Logs.
Simplify FDA/ISO compliance and accelerate daily workflows
with configurable modules and approvals.
and risk—now with Event & Hazard Logs.
Simplify FDA/ISO compliance and accelerate daily workflows
with configurable modules and approvals.
Choose your eQMS
eQMS
for MedDev
for MedDev
Compliant Quality Management
for Medical Devices
for Medical Devices
ISO 13485- and FDA-ready QMS.
Streamline document control, risk, CAPA and submissions with configurable workflows, e-signatures, and Event & Hazard Logs for audit-ready traceability.
Streamline document control, risk, CAPA and submissions with configurable workflows, e-signatures, and Event & Hazard Logs for audit-ready traceability.

eQMS
ISO 9001
ISO 9001
Simplify Your ISO 9001
Implementation
Implementation
ISO 9001 eQMS for continuous improvement.
Standardize processes, documents and CAPA with configurable workflows, e-signatures, and Event & Hazard Logs for end-to-end visibility.
Standardize processes, documents and CAPA with configurable workflows, e-signatures, and Event & Hazard Logs for end-to-end visibility.
All-in-One QMS Modules
qmsWrapper brings together 12 integrated modules that connect projects, documents, risks, CAPA, and suppliers into one system. With Event & Hazard Logs, every change and nonconformity is traceable, ensuring compliance without extra effort.
Compliance Made Simple
Stay audit-ready with automated workflows and clear traceability across all processes. From training to complaints, every record is linked and accessible—helping your team move faster while staying aligned with FDA and ISO requirements.