AI-Powered QMS for Medical Device Teams
The world's first AI-powered platform that unifies Design Control & Quality Event Management
Your Design Technical File (DTF) lives in one world. Your CAPAs, Deviations, and Non-Conformities live in another. Bridging them is manual, slow, and full of risk.
More than +500 companies trusted
qmsWrapper with their QMS.
qmsWrapper with their QMS.
Why qmsWrapper?
- Compliance Without Complexity
Meet FDA, ISO, MDR, HIPAA, and GDPR requirements with built-in modules, validated workflows, and audit-ready records.
- Workflow-Driven QMS Modules
Automate CAPAs, deviations, complaints, change controls, training, and more. Prebuilt flows keep you compliant—custom flows keep you in control.
- All-in-One Platform
Everything you need: Document control, electronic signatures, risk management, traceability, project and task tracking, custom forms, and audit trails.
- Build What You Need
Create unlimited custom modules, workflows, and topic-specific logs. Model any quality or operational process your team requires.
- Collaborate Without Switching Tools
Integrated messaging, task management, and project tracking keep conversations connected to actions.

What’s Included: Feature Categories
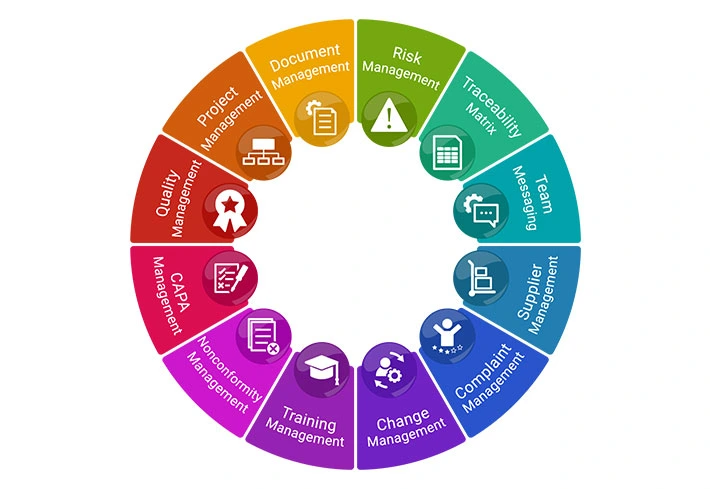
Core QMS & Compliance Modules
These support ISO 13485, ISO 9001, ISO 14971, FDA 21 CFR Part 820, FDA 21 CFR Part 11, EU MDR, IVDR, UK MDR/UKCA, MDSAP, and Health Canada regulations, as well as CE marking requirements.
- CAPA Management
- Deviation Management
- Nonconformity Management
- Change Management
- Customer Complaint / Feedback Management
- Training Management
- Supplier Management
- Audit Management
- Risk Management (ISO 14971)
- Hazard Logs
- Document Control with Versioning
- Electronic Signatures (21 CFR Part 11 compliant)
- Traceability Matrix
- Quality Manual Builder (with 80 Word Templates & 85 SOPs)
- Reporting Module
- Built-in Validation Package
Customization & Automation Tools
Give teams full flexibility to model and automate any quality process:
- Custom Module Builder – Create unlimited modules and topic-specific logs
- Form Builder – Design your own custom input forms
- Workflow Builder – Automate approvals, escalations, and reviews
- Prebuilt workflows for change control, NCs, complaints, training, and more
Collaboration & Productivity
Unify QMS and execution under one roof:
- Project Management Module
- Task/Issue/Event Management Module
- Conversation Module – Threaded, context-based internal communication
- Wrapper Desktop App – Real-time document editing and cloud sync
- Quick Access Dashboard for forms, tasks, and processes

Infrastructure & Support
Everything you need to stay productive, compliant, and secure:
- Secure Hosting with Daily Backup
- Windows/Mac/Linux Compatibility
- Role-Based Permissions
- Maintenance & Technical Support
- New Releases with Validated Updates
- API Integration with Jira

Onboarding & Customer Success
We guide you from setup to certification:
- Complimentary Onboarding & Training
- Setup Support
- Built-in Validation Documentation
- Fast Time-to-Compliance
Compliance Coverage
Comprehensive Compliance Support. qmsWrapper is built for global regulatory environments, helping your team stay compliant from day one:
Whether you're submitting a 510(k), preparing for ISO audits, or maintaining compliance for your SaMD across the US and EU, qmsWrapper is designed to support your journey.
- FDA 21 CFR Part 820 – Quality System Regulation
- FDA 21 CFR Part 11 – Electronic Records & Signatures
- FDA 510(k) – Submission-ready structure and traceability
- ISO 13485:2016 – Medical Devices QMS
- ISO 9001:2015 – General Quality Management
- ISO 14971:2019 – Risk Management for Medical Devices
- IVDR
- SaMD (Software as a Medical Device) and SaaS in regulated environments
- EU MDR / CE Marking
- UK MDR / UKCA
- GDPR – EU General Data Protection Regulation
- HIPAA – US Health Information Privacy and Security
Whether you're submitting a 510(k), preparing for ISO audits, or maintaining compliance for your SaMD across the US and EU, qmsWrapper is designed to support your journey.

Who Uses qmsWrapper?
qmsWrapper is trusted by teams across the MedTech space, including:
From your first audit to your tenth FDA or notified body submission, qmsWrapper brings control, clarity, and confidence to your compliance process.
- MedTech startups – Preparing for their first audit or FDA submission
- Medical device companies – Managing FDA 510(k), De Novo, CE, and UKCA submissions
- Diagnostics & biotech firms – Ensuring traceability, training, and risk management
- Contract manufacturers – Maintaining quality across multiple client projects
- Early-stage teams – Building a compliant QMS from day one
- ISO-certified companies – Maintaining ISO 13485, ISO 9001 with less overhead
- SaaS/Software as a Medical Device developers – Navigating IVDR and FDA regulations
- Quality teams – Who want to automate, not micromanage
From your first audit to your tenth FDA or notified body submission, qmsWrapper brings control, clarity, and confidence to your compliance process.
Your QMS. Your Way.
Easy to Use. Ready for Audits,
Submissions, and Growth.
qmsWrapper gives you everything you need—without the clutter. Unlike most bulky eQMS platforms, it’s lightweight, fast, and incredibly flexible.
With a clean, intuitive interface and modern user experience, your team actually wants to use it.
Whether you’re launching your first device, submitting a 510(k), or scaling globally, qmsWrapper simplifies compliance so you can move faster with confidence.